Moderna to test vaccine on infants

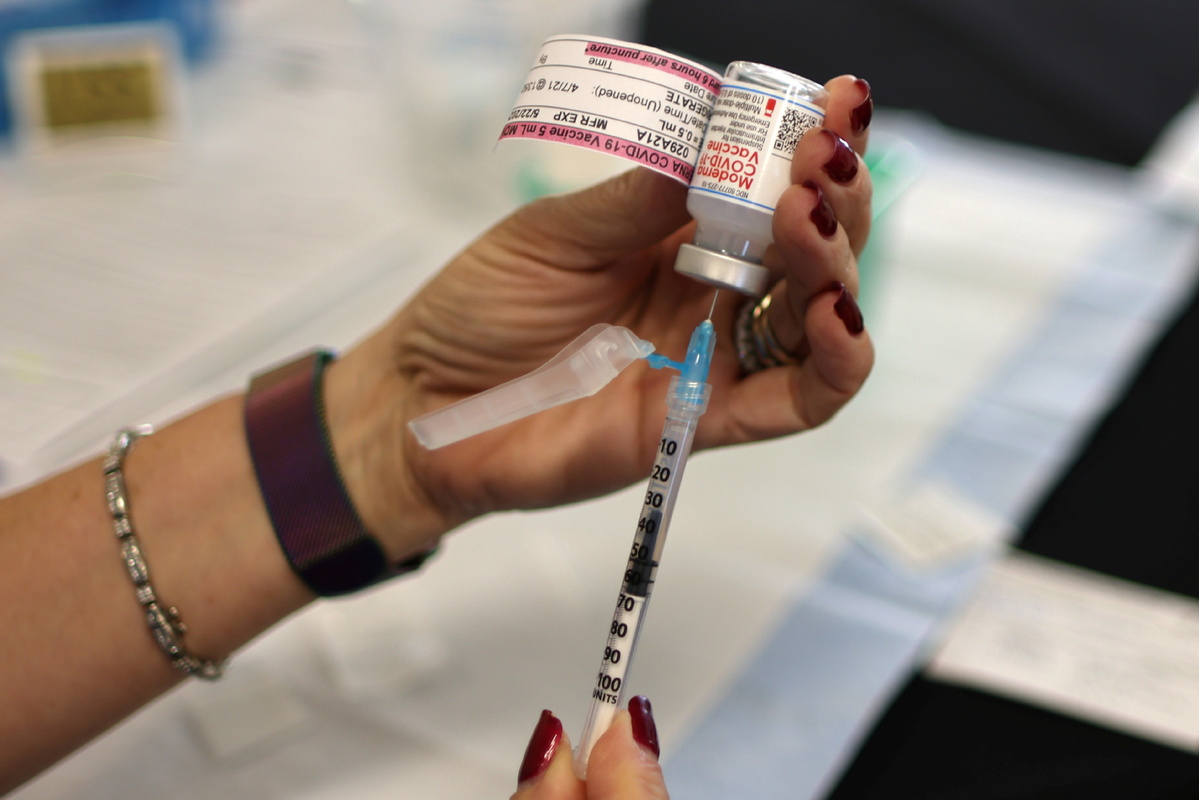
Moderna announced Tuesday that it will test its COVID-19 vaccine in children as young as 6 months, the first US vaccine maker to test on infants.
The clinical trial, named the KidCOVE, intends to enroll approximately 6,750 healthy children ages 6 months to 11 years in the US and Canada, the biotech company said.
The volunteer study will assess the safety and efficacy of Moderna's two-dose regimen, given 28 days apart. In a separate study, Moderna is testing its vaccine in 3,000 children ages 12 to 17 years and may have results for that age group this summer.
While only 216 children under age 17 have died of COVID-19, according to the latest Centers for Disease Control and Prevention data, children still can transmit the virus it to adults.
"We are encouraged by the primary analysis of the Phase 3 COVE study of mRNA-1273 in adults ages 18 and above and this pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger age population," said Stéphane Bancel, CEO of Moderna.
Moderna said that the first age group to start in the trial will be children ages 6 to 11, followed by children ages 2 to 6 and then children 6 months to 2 years of age.
The trial is the latest effort to widen the mass-vaccination campaign beyond adults. Most COVID-19 vaccines authorized in the US so far are protecting adults, who are at higher risk of severe disease caused by the coronavirus than children. Moderna's and Johnson & Johnson's vaccines are authorized for use in adults 18 and older, while the vaccine from Pfizer and BioNTech SE are for people 16 and older.
Moderna's trial comes as US schools start to reopen, and children are returning to classrooms under the guidelines of keeping 6 feet apart — or less — to avoid infections.
Dr Rochelle Walensky, CDC director, said the agency is now exploring whether children can be seated closer together than was previously recommended. The 6-feet spacing guideline is among the biggest challenges schools have faced in reopening, she said.
States including Illinois and Massachusetts are allowing 3 feet of distance, and others including Oregon are considering it.
The CDC suggested the 6-feet spacing limit in school guidelines issued last month, and concluded that schools can safely operate during the pandemic with masks, distancing and other precautions.
Other organizations have issued more relaxed guidelines. The American Academy of Pediatrics says to space desks "3 feet apart and ideally 6 feet apart". The World Health Organization urges 1 meter (3.28 feet).
Dan Domenech, executive director of AASA, a national school superintendents group, said he expects to see more states and schools move to the 3-feet rule in coming weeks. With the larger guideline, he said, most schools have the space to bring only half of their students in at a time. Moving to 3 feet could allow about 75 percent at a time, he said.
"There are districts that have been doing 3 feet for quite some time without experiencing any greater amount of infection," he said.
Meanwhile, encouraged by the rollout of COVID-19 vaccines, Americans are flying again.
More than 1.3 million people traveled through Transportation Security Administration checkpoints at airports both Friday and Sunday, according to data released by the government agency, setting a new high since the pandemic outbreak devastated travel a year ago.
"The fact that TSA checkpoint numbers continue to climb 5 percent every week underscores the optimism that travelers are feeling across the board," Scott Keyes, founder and chief flight expert at travel website Scott's Cheap Flights told MarketWatch. "With vaccinations accelerating and all adults eligible for the shot by May 1, their world will soon be all our world."
Southwest Airlines CEO Gary Kelly said during a Washington Post webcast that his airline could break even by June, "where you have had much of the population vaccinated".
President Joe Biden signed a $1.9 trillion pandemic relief bill on March 11, which includes $14 billion for airlines and an additional $9 billion for airports and other operations. The bill also sets aside $1 billion for aviation contractors and $8 billion for airports to help them operate normally, limit the spread of the virus, and pay workers and service their debts.
The stimulus package will protect airline jobs through Sep 30, as airlines must not furlough workers through then as a condition for receiving the aid. American Airlines and United Airlines told 27,000 employees that the warnings about furloughs received earlier are no longer valid.
Leading airline and business groups have asked the Biden administration to develop temporary credentials that would let travelers show they have been tested and vaccinated for COVID-19, but the White House said Monday the federal government shouldn't be involved in verifying that people have been vaccinated.
"It's not the role of the government to hold that data and to do that," Andy Slavitt, White House senior adviser for COVID-19 response, said in a news briefing.
While Americans need a way to reliably demonstrate that they've been vaccinated, the government shouldn't be the one issuing such a certification, he said.
"It should be private. The data should be secure. Access to it should be free. It should be available both digitally and in paper and in multiple languages. And it should be open source," Slavitt said.
"It is crucial to establish uniform guidance" and "the US must be a leader in this development," more than two dozen groups said in a letter last week to White House coronavirus-response coordinator Jeff Zients. However, the groups said that vaccination shouldn't be a requirement for domestic or international travel.
Various groups and countries are working on developing so-called vaccine passports aimed at allowing more travel. The US groups include the main US and international airline trade organizations, airline labor unions and the US Chamber of Commerce. The World Health Organization and the United Nations' aviation arm are working on the type of information to include in a credential.
Agencies contributed to this story.