Integration of TCM, modern medicine in the pipeline

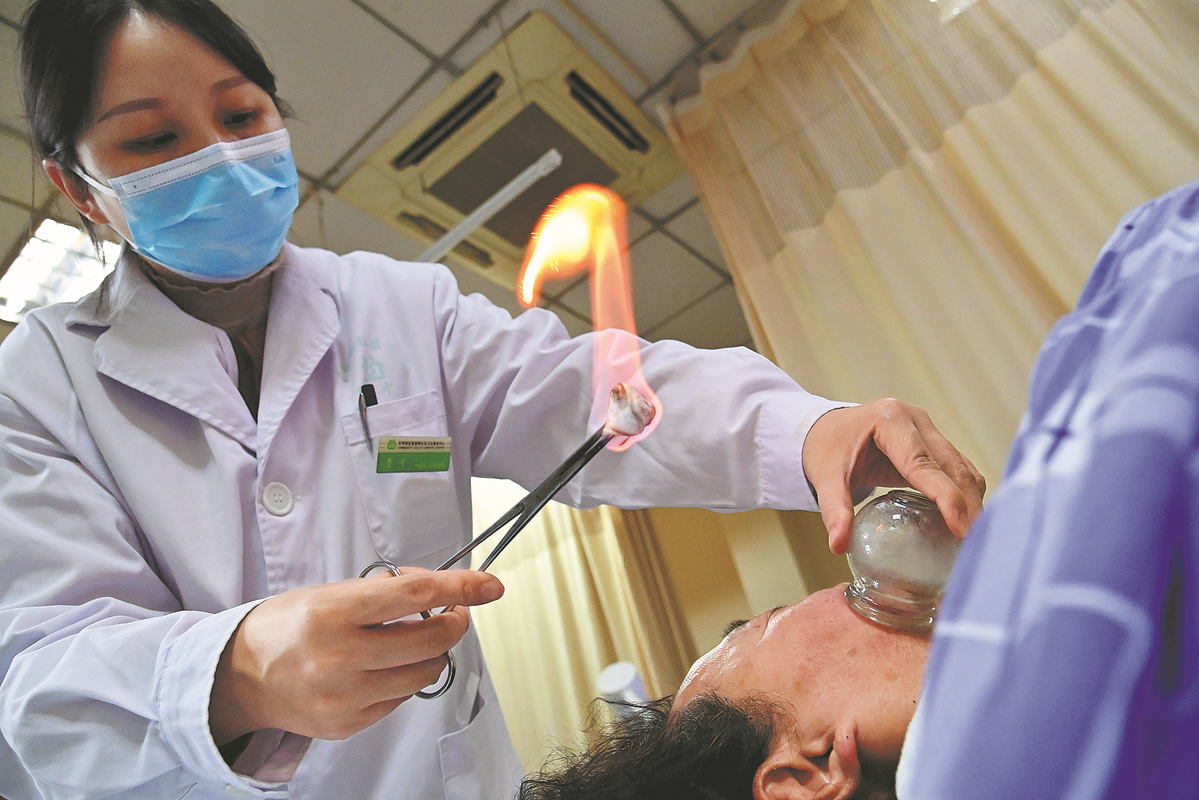
China is accelerating efforts to enhance the quality and innovation of traditional Chinese medicine, aiming to integrate modern science with ancient remedies while boosting market entry, officials said.
A new guideline to promote high-quality development of the TCM sector, recently issued by the State Council — the nation's Cabinet — emphasized both passed-down knowledge (inheritance), and innovation, they said at a news conference on Friday.
Lu Jianwei, deputy head of the National Administration of Traditional Chinese Medicine, said that the guideline underscores the application of digital and green technologies across the TCM supply chain, while boosting research and development for new medicines.
"Traditional Chinese medicine represents valuable heritage from our ancestors. We must preserve, develop and utilize it effectively," Lu said.
Chen Ronghu, director of the administration's science and technology department, said that TCM has great potential for original scientific breakthroughs. For example, artemisinin, a Nobel Prize-winning malaria treatment, and arsenic trioxide, which is used in leukemia therapy, are both derived from TCM principles.
To strengthen the research infrastructure, China has established seven key laboratories, five engineering research centers and four innovation platforms. Additionally, regional research hubs such as the Hengqin Lab in Guangdong province and the Haihe Lab in Tianjin contribute to a nationwide innovation network.
Meanwhile, a clinical research network has been established to evaluate TCM effectiveness.
"We will integrate artificial intelligence and big data to accumulate human-use experience and build a comprehensive clinical efficacy evaluation model to transform empirical knowledge into scientifically validated treatments and improve patient outcomes," Chen said.
The country is also advancing the integration of TCM into modern healthcare by optimizing its clinical use and expanding access to high-quality herbal medicines, he said, adding that efforts include developing standardized TCM formulations and increasing the availability of traditional herbal decoctions in hospitals and pharmacies nationwide.
Chen said China will focus on accelerating the development of innovative herbal medicines. The government plans to intensify research on complex TCM formulations, addressing major diseases and special medical needs such as the treatment of pediatric and chronic illnesses.
Yang Ting, head of the Department of Drug Registration at the National Medical Products Administration, said that China is speeding up the development and approval of new TCM treatments, with regulatory reforms boosting both innovation and market entry.
Applications for clinical trials and new drug approvals in the TCM sector have been steadily increasing, Yang said.
Last year, authorities accepted 100 clinical trial applications and 40 new drug applications, approving 12 new TCM products. Since the beginning of this year, eight new TCM drugs have already gained approval, marking a significant rise from the same period last year.
The NMPA has introduced a series of measures to accelerate TCM drug development, including streamlining drug review and approval processes while balancing tradition and innovation, Yang said.
"Unlike chemical or biological drugs, TCM formulations are deeply rooted in age-old medical practices. China has established a unique evidence-based evaluation system that combines TCM theory, human-use experience and modern clinical trials, shortening research timelines and increasing success rates of new drug development," he said.
Regulators have also improved communication channels with pharmaceutical companies, offering tailored guidance to facilitate the approval process.
"By providing clear guidance from the outset, we help developers avoid unnecessary obstacles, improving efficiency and effectiveness in bringing new TCM drugs to market," Yang said.
- Integration of TCM, modern medicine in the pipeline
- Forest food production key in agricultural output
- Ministry warns of rise in fire-risk areas
- Shenzhou XIX crew complete 3rd spacewalk
- SPA samples help analyze moon scar age
- China builds trans-scale biomedical imaging center to boost life sciences research